-Alloyours aluminum factory
In the intricate world of metallurgy, the distinction between ferrous and non-ferrous metals stands as a fundamental division. These categories not only define the elemental composition of metals but also shape their applications and properties. Amid this classification, one metal often sparks curiosity and intrigue: aluminium. Is it a ferrous metal, bound by the embrace of iron, or does it reside in the non-ferrous realm?
Is Aluminium A Ferrous Or Non Ferrous Metal?
Aluminium is unequivocally classified as a non-ferrous metal. This distinction arises from its elemental composition and intrinsic properties. Unlike ferrous metals, which prominently feature iron as a primary component, aluminium’s fundamental makeup sets it apart. As a chemical element with the symbol Al and atomic number 13, aluminium’s atomic structure and properties differ significantly from iron-based ferrous metals.
In this exploration, we delve into the depths of metallurgical science, peeling away the layers to unveil whether aluminium aligns itself with the ranks of non-ferrous metals or forges an unexpected connection with ferrous counterparts. Through scientific investigation, we seek to demystify aluminium’s true place in the intricate world of metal classification.
What is Ferrous and Non-Ferrous Metals?
To comprehend the classification of metals, it is essential to grasp the fundamental distinction between ferrous and non-ferrous metals. These categories form the bedrock of materials science and industry, shaping the behavior, properties, and applications of various metals. Ferrous and non-ferrous metals stand as two distinct realms, each with its own defining characteristics and implications.
Definition and Characteristics of Ferrous Metals
Ferrous metals, defined by their iron content, constitute a class of materials that exhibit distinct properties derived from the prevalence of iron atoms within their atomic structure. The presence of iron lends ferrous metals several key characteristics that set them apart from their non-ferrous counterparts.
Iron’s atomic arrangement contributes to the strong intermolecular forces within ferrous metals, resulting in exceptional tensile strength. This quality makes ferrous metals a preferred choice in applications requiring structural integrity and the ability to withstand substantial mechanical stress.
The crystalline structure of iron grants ferrous metals magnetic properties. These metals can be readily magnetized and retain their magnetization even after the magnetic field is removed. This attribute has significant implications in industries such as electronics and machinery.
The inherent strength of ferrous metals imparts durability, enabling them to endure harsh environmental conditions and resist deformation under external forces. This makes ferrous metals an ideal choice for applications that demand longevity and robustness.
While ferrous metals possess remarkable mechanical attributes, their iron content renders them susceptible to corrosion when exposed to moisture and oxygen. Rust formation is a common challenge, necessitating protective coatings or treatments to enhance their resistance to oxidation.
Iron’s affinity for alloying with other elements allows for the creation of a diverse range of ferrous alloys. These alloys exhibit tailored properties, making them suitable for specific applications. Examples include stainless steel, which combines iron with chromium for enhanced corrosion resistance, and cast iron, valued for its exceptional casting properties.
In summary, ferrous metals stand as a group characterized by their iron-dominated composition and the resulting attributes they inherit. Their high tensile strength, magnetic properties, durability, and alloying potential position them as versatile materials with applications spanning from construction and manufacturing to automotive and engineering, illustrating the intricate interplay of chemistry, physics, and engineering within the realm of metallurgy.
Definition and Characteristics of Non-Ferrous Metals
Non-ferrous metals encompass a diverse group of elements that lack a significant presence of iron within their composition. This absence of iron imparts unique properties that distinguish them from their ferrous counterparts. Let’s delve into the defining characteristics of non-ferrous metals:
Non-ferrous metals include a wide array of elements, such as copper, aluminum, zinc, lead, and more. Each element contributes distinct properties to the resulting metal, making non-ferrous metals versatile and adaptable for various applications.
Many non-ferrous metals excel in conducting electricity, owing to their atomic structure and electron mobility. Copper, for instance, is renowned for its exceptional electrical conductivity, making it an essential component in wiring and electrical systems.
Non-ferrous metals are generally more resistant to corrosion than their ferrous counterparts due to the absence of iron. Elements like aluminum naturally form protective oxide layers that shield the metal from environmental elements, prolonging their lifespan.
Non-ferrous metals often have lower densities than ferrous metals, resulting in lightweight materials. Aluminium, in particular, stands out for its remarkable lightweight properties, making it a staple in aerospace, automotive, and packaging industries.
The lack of iron in non-ferrous metals contributes to their non-magnetic nature. This property finds applications in industries where magnetic interference must be minimized, such as electronics and medical equipment.
Many non-ferrous metals exhibit excellent malleability and ductility, allowing them to be shaped into intricate forms without fracturing. This property is advantageous in applications requiring intricate designs and complex geometries.
In summary, non-ferrous metals encompass a diverse range of elements with distinct properties that render them indispensable in various industries. Their electrical conductivity, corrosion resistance, lightweight nature, and malleability make them essential components in applications ranging from electronics and construction to aerospace and renewable energy, reflecting the nuanced interplay between atomic properties and real-world functionality.
Aluminium's Elemental Identity
Nestled within the periodic table with atomic number 13, aluminium beckons us to explore its elemental identity. As we dissect its atomic structure and delve into its unique properties, a clearer understanding emerges of why aluminium’s allegiance lies firmly within the realm of non-ferrous metals.
Aluminium, denoted by the symbol Al, boasts a unique set of chemical properties that distinguish it from ferrous metals. It forms a protective oxide layer upon exposure to air, effectively shielding the metal from corrosion. This oxide layer, aluminium oxide (Al2O3), is exceptionally stable and contributes to the metal’s impressive corrosion resistance. This property, amplified through meticulous processing by the aluminum factory, is a hallmark of aluminium’s versatility in applications ranging from construction to electronics.
Atomic Structure
Delving deeper, we encounter aluminium’s atomic structure—an arrangement of 13 protons, 13 electrons, and a variable number of neutrons in its nucleus. This composition lends aluminium its characteristic properties, including its lightweight nature and remarkable conductivity. The alignment of electrons in energy levels within the atomic structure determines aluminium’s interactions with external stimuli, influencing its behavior in various environments.
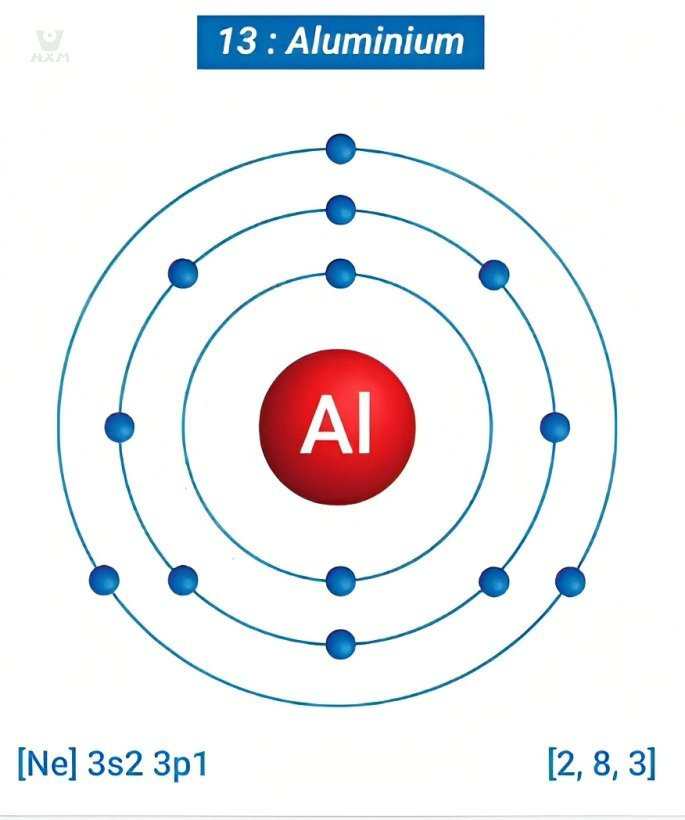
Through the lens of chemical properties and atomic structure, we begin to decipher the intricate story of aluminium’s non-ferrous identity. The aluminum factory role in refining its properties underscores the meticulous science behind this metal’s journey from raw material to industrial cornerstone. Join us as we unravel the scientific nuances that position aluminium as an elemental marvel, shaping industries and technologies across the globe.
why aluminium does not belong to ferrous metals?
Aluminium’s distinct elemental identity emerges from its composition and inherent properties, setting it apart from the ferrous metals group. A scientific analysis, guided by the expertise of the aluminum factory, reveals the core reasons for aluminium’s exclusion from the realm of ferrous metals.
At the heart of this differentiation lies aluminium’s elemental composition. While ferrous metals, by definition, contain iron as a major constituent, aluminium’s fundamental composition lacks a significant presence of iron atoms. Aluminium’s atomic number 13 signifies its unique arrangement of protons, neutrons, and electrons, conferring upon it a distinct set of properties that stand in contrast to those of iron-dominated ferrous metals.
Aluminium’s atomic structure deviates from that of ferrous metals like iron and steel. The arrangement of electrons in its energy levels, along with its atomic size and bonding characteristics, grants aluminium properties such as lightweight nature, corrosion resistance, and excellent electrical conductivity. These properties are not shared by ferrous metals, which exhibit distinct behaviors rooted in their iron-rich atomic makeup.
One of the most significant distinctions between aluminium and ferrous metals is the former’s non-magnetic nature. Unlike ferrous metals, which are inherently magnetic due to iron’s influence, aluminium remains impervious to magnetic forces. This property underscores aluminium’s departure from the ferromagnetic realm and further emphasizes its non-ferrous classification.
Through this scientific lens, we unveil the intrinsic reasons why aluminium finds its place among non-ferrous metals. Its elemental composition, atomic structure, and contrasting properties form a compelling narrative that reinforces aluminium’s status as a remarkable non-ferrous element. The aluminum factory expertise in refining these properties underscores the precision science behind aluminium’s characterization and its pivotal role across diverse industries.
Aluminium vs. Ferrous Metals
In the world of metallurgy, the division between aluminium and ferrous metals is more than just a matter of elemental composition—it’s a story of contrasting properties, behaviors, and applications. As we journey deeper into the realm of metals, guided by the insights of the aluminum factory, we confront the stark differences that define aluminium’s departure from the iron-dominated landscape of ferrous metals.
Physical Properties
- Density and Weight: Aluminium, with its lower density compared to most ferrous metals, is notably lightweight. This attribute makes it a preferred choice in industries where weight reduction is crucial, such as aerospace and automotive manufacturing. Ferrous metals, on the other hand, often exhibit higher densities due to their iron content, contributing to their robustness in applications requiring structural integrity.
- Strength and Hardness: Ferrous metals, known for their iron-rich composition, tend to possess higher tensile strength and hardness. Steel, a prominent ferrous metal, gains its renowned strength from iron's participation in its molecular structure. Aluminium, while not as inherently strong, compensates with its impressive lightweight strength, suitable for applications where a balance between strength and weight is essential.
- Thermal Conductivity and Melting Point: Aluminium's excellent thermal conductivity is a notable feature, allowing it to efficiently dissipate heat. This property is integral to its use in heat sinks and various cooling applications. In contrast, ferrous metals often have lower thermal conductivity compared to aluminium. Additionally, aluminium's lower melting point compared to most ferrous metals makes it more amenable to casting processes.
- Electromagnetic Properties: Ferrous metals, due to their iron content, generally exhibit magnetic behavior. In contrast, aluminium remains non-magnetic, a characteristic derived from its elemental composition. This non-magnetic property contributes to its utilization in electronic devices and applications where electromagnetic interference must be minimized.
- Corrosion Resistance: Aluminium's natural oxide layer grants it remarkable corrosion resistance. This oxide layer forms quickly upon exposure to air, protecting the metal from environmental factors. Ferrous metals, while susceptible to corrosion due to iron's reactivity, often require additional coatings or treatments to enhance their resistance.
Through this comparison of physical properties, guided by insights from the aluminum factory, we discern the divergent attributes that define aluminium and ferrous metals. Each metal’s distinctive characteristics dictate their respective roles in industries, shaping the landscape of modern engineering, design, and innovation.
Industrial Applications
- Aerospace and Automotive Industry: Aluminium, celebrated for its lightweight nature, finds a natural home in the aerospace and automotive sectors. Its low density contributes to fuel efficiency and overall performance, enabling aircraft to soar higher and vehicles to consume less energy. In contrast, ferrous metals like steel lend their robustness to automotive frames and structural components, emphasizing durability and safety.
- Construction and Infrastructure: Both aluminium and ferrous metals play pivotal roles in the construction industry. Aluminium's corrosion resistance and malleability make it an asset for architectural applications, while ferrous metals provide the strength needed for bridges, skyscrapers, and reinforcing concrete structures. The choice between these metals hinges on the specific demands of the project.
- Electronics and Technology: Aluminium's excellent electrical conductivity and lightweight properties make it a staple in electronics. It dissipates heat effectively, prolonging the lifespan of electronic devices. Ferrous metals, however, offer magnetic shielding, rendering them valuable in cases where electromagnetic interference must be mitigated.
- Packaging and Consumer Goods: Aluminium's impermeability to moisture and gases, along with its corrosion resistance, makes it an ideal choice for packaging perishable goods and beverages. Its lightweight nature also contributes to energy-efficient transportation. In contrast, ferrous metals may be utilized in packaging machinery due to their durability and magnetic properties.
- Renewable Energy and Infrastructure: Both aluminium and ferrous metals contribute to renewable energy systems. Aluminium's corrosion resistance and durability make it suitable for solar panels and wind turbine components. Ferrous metals, due to their magnetic properties, are utilized in generators and electromagnets in energy production.
Through these industrial applications, influenced by the expertise of the aluminum factory, we witness the distinct roles that aluminium and ferrous metals play in shaping industries. Their unique properties influence design, efficiency, and durability, underscoring the significance of selecting the right material for the right purpose.
Environmental Sustainability
- Environmental Impact and Recycling: Aluminium boasts a notable advantage in terms of environmental sustainability due to its high recyclability rate. Recycling aluminium requires significantly less energy compared to primary production, reducing greenhouse gas emissions and energy consumption. The expertise of the aluminum factory plays a crucial role in efficiently reclaiming and reprocessing aluminium for various applications. Ferrous metals, although also recyclable, may require more energy-intensive processes for extraction and recycling.
- Resource Availability: Aluminium derives from bauxite ore, which is abundant globally. This relatively widespread availability minimizes concerns over resource scarcity. Ferrous metals, while iron is abundant, may face resource challenges due to variations in ore quality and accessibility.
- Life Cycle Assessment: Life cycle assessments (LCAs) evaluate the environmental impact of materials throughout their entire life cycle. Aluminium's lightweight nature contributes to reduced energy consumption during transportation, lowering its carbon footprint. Additionally, the ease of recycling aluminium further enhances its positive environmental profile. Ferrous metals, depending on the specific alloy and application, may require more energy during extraction, production, and transportation, affecting their overall environmental impact.
- Corrosion Resistance and Longevity: Aluminium's natural corrosion resistance extends the lifespan of products, reducing the need for frequent replacements. This attribute aligns with sustainable practices by conserving resources and minimizing waste. Ferrous metals, susceptible to corrosion, may require protective coatings to enhance their durability and longevity.
In the realm of environmental sustainability, as guided by insights from the aluminum factory, aluminium emerges as a contender with distinct advantages. Its recyclability, resource availability, and extended product lifespan underscore its role in promoting eco-friendly practices. While ferrous metals contribute to recycling efforts, their specific characteristics may warrant careful consideration to achieve optimal environmental outcomes.
conclusion
In conclusion, the exploration of aluminium’s elemental identity unveils its classification as a non-ferrous metal, rooted in its composition, atomic structure, and inherent properties. This distinction sets aluminium on a distinct trajectory, diverging from the iron-rich landscape of ferrous metals. Its non-magnetic nature, lightweight strength, and corrosion resistance define its essence, making it a prized material across diverse industries.
Role of the Aluminum Factory
The recognition of aluminium as a non-ferrous metal holds significance beyond semantics. Understanding this distinction illuminates the unique properties that drive aluminium’s applications, from aerospace innovation to sustainable packaging. It underscores the intricate relationship between atomic composition and real-world functionality. The expertise of the aluminum factory further accentuates the precision science required to refine aluminium’s properties for tailored applications.
By appreciating the intrinsic qualities that set aluminium apart, we gain insights into the vast potential of materials science. This exploration reminds us that the path to innovation is paved with a nuanced understanding of the elements, their properties, and their transformative capabilities across industries. As we navigate the realm of metals, the dichotomy between ferrous and non-ferrous—exemplified by aluminium—exemplifies the profound influence that these classifications hold in shaping our modern world.







