When it comes to the world of metals, aluminum stands out as a true icon of modern engineering and innovation. Its lightweight yet robust nature has revolutionized industries, from aviation to construction, making it an indispensable material in our daily lives. But here’s a question that often arises: Is aluminum magnetic?
Is Aluminum Magnetic?
No, aluminum is not magnetic in the traditional sense. It is considered a non-magnetic metal due to its atomic structure. While aluminum does interact with magnetic fields, its response is very weak. This is because aluminum’s electrons are arranged in a way that doesn’t result in a significant alignment of magnetic moments, which is necessary for strong magnetism.
In this blog, we embark on a journey to demystify the magnetic characteristics of aluminum. We’ll explore the fundamental science behind magnetism, delve into the atomic structure of aluminum, and reveal the secrets that make certain metals magnetic while leaving others unaffected. Whether you’re an engineer seeking the perfect material for your project or simply a curious mind eager to understand the science behind everyday materials, this blog will serve as your guide to understanding the magnetic allure – or lack thereof – of aluminum.
The Basics of Magnetism
Magnetism, one of the fundamental forces of nature, has a mesmerizing influence on our everyday lives. From the gentle pull of a refrigerator magnet to the powerful forces steering Earth’s magnetic field, its presence is both intriguing and indispensable. In our exploration of whether aluminum is magnetic, we must first understand the basic principles of magnetism that govern the behavior of materials.
Brief Overview of Magnetism and Its Types: Navigating the Magnetic Spectrum
Magnetism, a captivating force that has intrigued scientists and thinkers for centuries, arises from the alignment and motion of charged particles. This force is responsible for the attractive or repulsive interactions between materials and has been harnessed for a wide range of applications.
There are three primary types of magnetism: ferromagnetism, paramagnetism, and diamagnetism.
Ferromagnetism is synonymous with strong and enduring magnetism. In ferromagnetic materials such as iron, nickel, and cobalt, clusters of atoms align their magnetic moments in the same direction, creating domains with a net magnetic alignment. When an external magnetic field is applied, these domains align, resulting in a macroscopic magnetization. Even without an external field, ferromagnetic materials retain their magnetism, making them ideal for applications like permanent magnets and magnetic storage media.
Paramagnetic materials are characterized by their temporary attraction to magnetic fields. Within these materials, certain atoms or ions possess unpaired electrons, causing them to align their spins with an applied field. This alignment is short-lived and disappears when the field is removed. Aluminum, among others, falls into this category. While paramagnetism is weaker than ferromagnetism, it’s still a significant effect and finds use in applications like MRI machines and electronic components.
Diamagnetic materials, including water, wood, and most organic compounds, exhibit a unique behavior. When subjected to a magnetic field, their atoms generate tiny opposing magnetic fields, leading to a weak repulsion. Diamagnetic effects are generally subtle and often overshadowed by other types of magnetism. However, they’re responsible for the levitation of certain materials in strong magnetic fields, a phenomenon observed in various experimental setups.
Understanding these magnetic personalities is crucial in deciphering aluminum’s magnetic behavior. While aluminum isn’t ferromagnetic, its paramagnetic nature plays a role in its interaction with magnetic fields. As we delve deeper into the atomic structure of aluminum, we’ll gain insights into why its magnetic response is distinct from its ferromagnetic and diamagnetic counterparts.
Aluminum's Atomic Structure
At the heart of every material’s properties lies its atomic structure – an intricate dance of electrons, protons, and neutrons that shapes how the material behaves on a macroscopic level. Aluminum, a ubiquitous element in our modern world, is no exception. To understand why aluminum behaves the way it does in the presence of magnetic fields, we must embark on a journey into its atomic realm.
Aluminum's Atomic Arrangement
Aluminum, a lightweight and versatile metal cherished by industries worldwide, owes its remarkable properties to the intricacies of its atomic structure. At the heart of every atom lies a nucleus composed of protons and neutrons, surrounded by a cloud of electrons that dictate the material’s behavior.
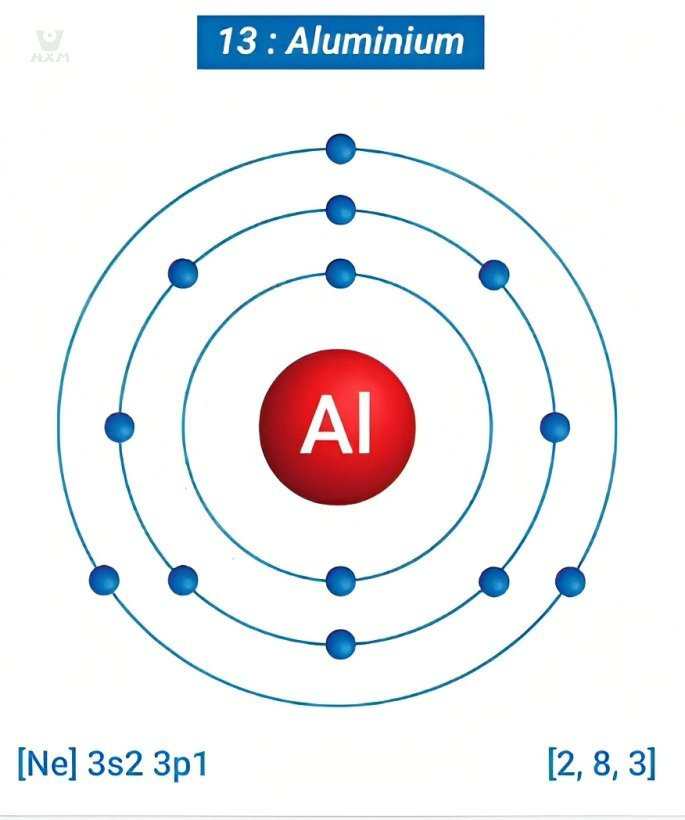
In aluminum’s case, each atom boasts 13 electrons, each with its own unique energy level and orbital pattern. These electrons fill successive energy levels, with two in the innermost shell, eight in the second, and the remaining three in the outermost shell. This arrangement bestows aluminum with its characteristic properties, enabling it to efficiently conduct electricity, exhibit high reflectivity, and maintain exceptional corrosion resistance.
As aluminum manufacturers, comprehending this atomic arrangement is vital. It’s this very arrangement that underpins aluminum’s non-magnetic nature. The arrangement of electrons in its outermost shell prevents the formation of strong magnetic moments, differentiating it from ferromagnetic materials where such alignment leads to robust magnetism.
By delving into the realm of atoms and their quantum behaviors, we uncover the foundation of aluminum’s unique characteristics. This understanding allows us to harness aluminum’s strengths for a multitude of applications across industries.
Electron Configuration and Magnetic Moments
The magnetic properties of a material are intricately linked to the behavior of its electrons – those tiny, charged particles that dance around atomic nuclei. For aluminum manufacturers seeking to understand why aluminum remains non-magnetic, delving into electron configuration and magnetic moments is essential.
Aluminum’s electron configuration plays a pivotal role in its magnetic behavior. As we said before, with 13 electrons, aluminum’s innermost and second energy levels are fully occupied with 2 and 8 electrons respectively. This leaves 3 electrons in its outermost shell, creating a configuration of 2-8-3. This configuration leaves aluminum without unpaired electrons in its outermost shell, a characteristic trait of magnetic materials.
Magnetic moments, arising from the spin and orbital angular momentum of electrons, dictate a material’s magnetic behavior. In ferromagnetic materials, the alignment of these moments leads to a strong collective magnetization. In aluminum, however, the pairing of electrons in its outer shell results in opposing magnetic moments that cancel each other out. This absence of aligned moments is what renders aluminum non-magnetic.
For aluminum manufacturers, grasping the interplay between electron configuration and magnetic moments is pivotal. It not only explains aluminum’s lack of ferromagnetism but also highlights its paramagnetic nature. While aluminum does interact with magnetic fields, its weak magnetic response is a testament to the delicate balance between paired and unpaired electrons.
How Atomic Structure Affects Magnetic Behavior?
The intricate dance of electrons within an atom orchestrates the magnetic symphony of materials. As aluminum manufacturers seeking to comprehend the essence of aluminum’s non-magnetic character, we must delve into how its atomic structure orchestrates its magnetic behavior.
The arrangement of electrons in their energy levels and shells directly influences a material’s magnetism. In aluminum’s case, the filled innermost and second energy levels, coupled with the partially filled third level, result in an electron configuration that lacks unpaired electrons. This unique configuration is pivotal – it’s what prevents aluminum from becoming a ferromagnet like iron.
Magnetic moments, borne from the spin and movement of electrons, play a decisive role. In ferromagnetic materials, unpaired electrons align their spins, resulting in a net magnetization. In aluminum, however, its electron arrangement leads to opposing magnetic moments that largely cancel each other out, yielding weak magnetism at best.
For aluminum manufacturers, this understanding holds the key to optimizing aluminum’s applications. The absence of strong magnetism makes aluminum a valuable asset in fields where magnetic interference is detrimental. This trait, rooted in its atomic structure, shapes aluminum into an invaluable material for industries such as electronics and construction.
Aluminum's Weak Magnetic Response
While aluminum is not considered a magnetic material in the conventional sense, its interactions with magnetic fields are far from insignificant. In this section, we will delve into the intricacies of aluminum’s magnetic behavior, dissecting the factors that contribute to its subtle attraction.
From the vantage point of aluminum manufacturers, this understanding offers a strategic edge. By comprehending aluminum’s unique relationship with magnetism, we can better harness its properties for various applications, from electrical systems to architectural designs. Join us as we peer into the realm of magnetic interactions and decipher the puzzle of aluminum’s magnetic identity.
Aluminum's Interaction with Magnetic Fields
Aluminum, known for its exceptional versatility and widespread industrial use, reveals a nuanced dance when introduced to magnetic fields. As aluminum manufacturers, understanding this interaction provides us with a deeper grasp of aluminum’s behavior and its implications for various applications.
When exposed to a magnetic field, aluminum experiences a phenomenon known as paramagnetism. While not as robust as ferromagnetism, this interaction is far from trivial. Paramagnetic materials, like aluminum, possess unpaired electrons in their atomic structure. When placed in a magnetic field, these unpaired electrons align their spins with the field’s direction, creating a subtle attraction.
However, it’s vital to note that aluminum’s weak magnetic response doesn’t lead to magnetization that persists after the magnetic field is removed. Unlike ferromagnetic materials that retain their magnetism, aluminum’s magnetic alignment quickly dissipates once the external field is taken away.
For aluminum manufacturers, this interaction has intriguing implications. While aluminum’s paramagnetic nature doesn’t make it a magnetic material in the traditional sense, it does play a role in fields where magnetic interference needs to be minimized. This attribute enhances aluminum’s value in industries like electronics and telecommunications.
By unraveling the intricacies of aluminum’s interaction with magnetic fields, we unlock new dimensions of its utility. This knowledge empowers us to make informed decisions when choosing materials for diverse applications, ensuring that aluminum’s magnetic behavior aligns seamlessly with project requirements.
Comparison to Ferromagnetic Metals
In the magnetic tapestry of metals, aluminum stands as a unique entity, distinct from its ferromagnetic counterparts such as iron and nickel.
Ferromagnetic metals like iron and nickel exhibit robust and enduring magnetism. The alignment of their atomic magnetic moments leads to the creation of domains that contribute to strong magnetization even in the absence of external magnetic fields. This intrinsic magnetism underpins applications ranging from motors to data storage.
On the other hand, aluminum’s magnetic identity is characterized by paramagnetism, a subtle interaction where unpaired electrons align with an applied magnetic field. While this response is weaker than ferromagnetism, it’s enough to evoke noticeable attraction. Yet, what sets aluminum apart is its swift return to a non-magnetic state once the magnetic field is removed.
For aluminum manufacturers, this distinction holds practical significance. The absence of ferromagnetism in aluminum allows for applications where magnetic interference needs to be minimized. Its lightweight, non-magnetic nature renders it invaluable in aerospace, electronics, and environments where precise magnetic control is imperative.
Aluminum's Minimal Attraction to Magnets
In the realm of magnetism, a simple experiment can reveal the intriguing nature of aluminum’s magnetic response. As aluminum manufacturers seeking to understand its properties, this demonstration sheds light on aluminum’s unique behavior in the presence of magnetic fields.
Place a strong magnet near a piece of aluminum and observe the interaction – or lack thereof. Unlike ferromagnetic materials that would rush to attach themselves, aluminum exhibits minimal attraction. This is a testament to its paramagnetic nature, where the unpaired electrons in its atomic structure respond to the magnetic field, causing a faint pull.
However, this attraction is a far cry from the powerful pull seen with ferromagnetic materials like iron. And perhaps even more intriguingly, remove the magnet, and aluminum promptly lets go – a hallmark of paramagnetic behavior. This swift return to a non-magnetic state highlights the dynamic and reversible nature of aluminum’s interaction with magnetic fields.
For aluminum manufacturers, this simple demonstration offers valuable insights. It showcases how aluminum’s weak magnetic response can be harnessed for applications where magnetic interference must be minimized. From electronic devices to precision instruments, aluminum’s non-magnetic quality opens doors to innovative solutions.
Factors Influencing Magnetism
In the realm of magnetism, multiple factors converge to shape a material’s behavior. For aluminum manufacturers, comprehending these dynamics is pivotal in harnessing aluminum’s properties effectively.
Role of Unpaired Electrons in Determining Magnetic Properties
The magnetic identity of a material hinges on its electron configuration. In aluminum’s case, the presence of unpaired electrons in its outermost shell contributes to its paramagnetic behavior. These unpaired electrons align with an applied magnetic field, resulting in a subtle attraction. Understanding this electron-level interplay allows aluminum manufacturers to anticipate how aluminum will respond in various magnetic scenarios.
Effects of Temperature on Magnetism and Curie Temperature
Temperature has a profound impact on a material’s magnetism. Ferromagnetic materials exhibit a critical temperature called the Curie temperature, beyond which their magnetism fades. Aluminum manufacturers need to be aware that aluminum lacks a Curie temperature, remaining paramagnetic even at low temperatures. This property holds significance in applications that involve extreme environments, where maintaining magnetic stability is crucial.
Magnetic Susceptibility
Magnetic susceptibility quantifies a material’s responsiveness to a magnetic field. Aluminum’s low magnetic susceptibility is a hallmark of its paramagnetic nature. It’s essential for aluminum manufacturers to recognize this characteristic when selecting materials for applications where magnetic interactions must be precisely controlled or minimized.
By understanding these factors, aluminum manufacturers gain a holistic view of aluminum’s magnetic behavior. This knowledge aids in tailoring aluminium’s applications across diverse industries, from electronics to aerospace, ensuring that its magnetic traits align harmoniously with project requirements.
Conclusion
In the realm of materials science, aluminum emerges as a fascinating enigma when it comes to magnetism. As aluminum manufacturers, this journey into understanding aluminum’s magnetic behavior equips us with insights that transcend mere curiosity.
Throughout our exploration, we’ve deciphered the intricate science behind aluminum’s magnetic response. It’s clear that while aluminum interacts with magnetic fields, its paramagnetic nature sets it apart from ferromagnetic materials. The absence of unpaired electrons in its outer shell denies aluminum the strong magnetic moments characteristic of magnetic metals.
Aluminum’s unique non-magnetic qualities make it a valuable asset across a spectrum of industries. From electronics to construction, aerospace to automotive, its lightweight, corrosion-resistant, and non-interfering characteristics find applications in countless innovations. As aluminum manufacturers, embracing aluminum’s distinctiveness empowers us to deliver cutting-edge solutions.







